A. Cells control the movement of every part of their body.
Cell movement is not random.. The cortex consists of autonomous domains ('microplasts') whose movement is controlled by a control center (centrosome). Microtubules mediate between the controll center and the autonomous domains.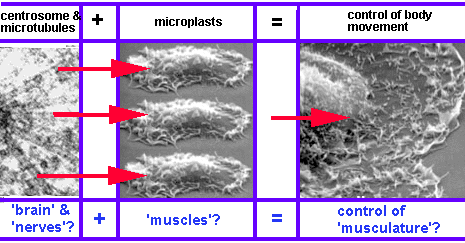
B. The control center detects objects and other cells objects by pulsating near-infrared signals.
Cell have 'eyes' in the form of centrioles.. They are able to detect near infrared signals and steer the cell movements towards their source.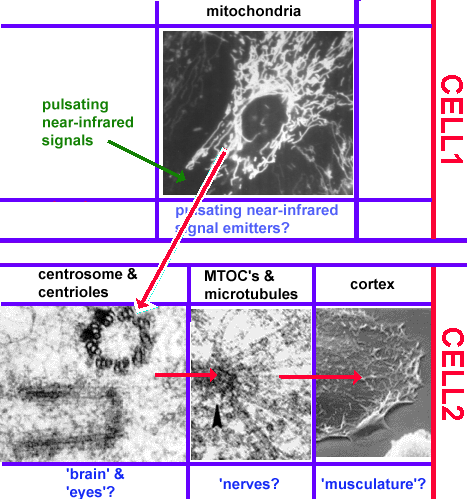
For the past 2 decades I have applied essentially two lines of reasoning to examine whether cells are intelligent. They can be summarized in the following statements:
A. If cells can measure space and time, they must be able to derive abstract data from physical signals.
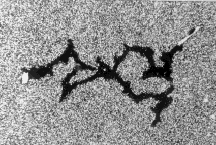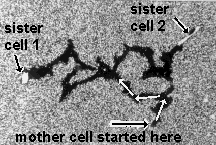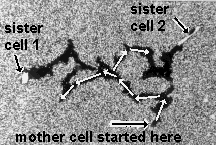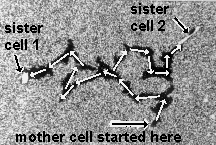
Space and time are not physical objects with which cells could interact, but they are the pre-condition of all physical objects. If cells can measure space and time variables such as angles, distances, curvatures or durations, they must have derived these abstract quantities from the physical objects of their environment. Chapter 2 will use the apparent symmetry and identity between the branches of the phagokinetic tracks of dividing cells (an example is shown below) to argue that cells are programmed to measure angles and time durations.

B. If cells have eyes, they must be able to order and integrate countless signals.
Images are the ordered set of a huge number of individual data. If cells are capable of generating an image of their environment and react to it, they must be able to order a large number of signals and integrate them into a response action. Chapter 3 will present the evidence that cells use centrioles to 'see' all objects around them that emit or scatter near infrared light. The figure below shows one of the examples of this amazing ability of cells.
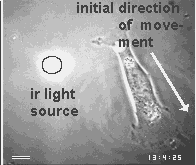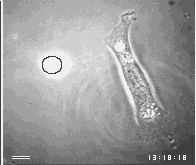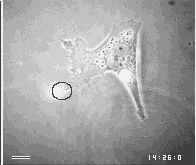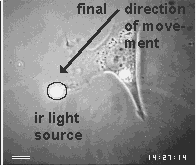
First a disclaimer. My work over the past 2 decades did not intend to join the ongoing efforts of philosophers, logicians and computer scientists to find a universal definition of intelligence. On the contrary, it did not question the common assumption that everybody can tell a mindless, mechanical gadget from an intelligent machine, and proceeded to ask which of the two categories apply to a living cell. Clearly, there are many different levels of intelligence, but I believe that most people consider a machine mechanical and mindless if its actions either do not seem to respond to signals or else always show the same set of reactions. On the other hand, we expect an intelligent machine to responds to signals in a large variety of ways, especially if the signals are unforeseeable, and if its responses offer solutions to problems which were transmitted by the signals. Usually, this means that the intelligent machine contains at least 2 different machines, one which it mindless and carries out some mechanical labor while the other collects and processes signals and controls the action of the first.
Therefore, I went ahead using the following operational definition of an intelligent cell. An intelligent cell contains a compartment which is capable of collecting and integrating a variety of physically different and unforeseeable signals as the basis of problem-solving decisions.
The prevailing wisdom of modern biology has it that cells are immensely complex, but rigidly operating chemical machines that derive their operating instructions internally from their genes and externally from chemicals and electrical signals emitted rigidly by other cells. Unable to believe that any machine can be designed that contains an instruction library which anticipates all the mishaps and glitches of a billion years of evolution without crashing over and over again, I began more than 2 decades ago to search for signs that the cell was actually a 'smart' machine. In other words, I looked for experimental evidence that cells contained a signal integration system that allowed them to sense, weigh and process huge numbers of signals from outside and inside their bodies and to make decisions on their own.
One of the reasons for my disbelief was the sheer size of the organisms that cells built out of themselves. Organism like us are 30,000 times larger in length and and we are not the biggest. Keep in mind that the cells of a gnat are not smaller than the cells of a whale. The whale just contains a lot more cells. How could they build such a huge range of organisms without the ability to override any permanent instructions in order to solve unforeseeable problems on its own?
Anybody who ever built anything bigger than himself knows what frightening surprises it can bring to exceed the dimensions of actions and objects with which we are familiar. The Sears Tower in Chicago is 'only' 300 times larger than the body of its architects. Ask the architects and construction companies who solved the legions of surprise technical problems whether an architect rigidly programmed to respond mechanically could have built it! Then try to build something 30,000 times larger, i.e. a building which is 25 miles tall with a rigid and mechanically operating robot!
Under what circumstances would a cell reveal that it is 'intelligent'? I thought that the best place to start searching was the field of cell movement. A moving cell has to operate its own body in sophisticated ways and, in addition, may have to navigate in space and time while dealing with numerous unforeseeable events, such as encounters with other cells and other objects that its genome could not possibly have anticipated.
I think that cell motility, indeed, revealed cell intelligence. This website highlights some of the experiments and offers the images and the arguments that support the claim of cell intelligence. The left side of the screen guides the visitor to the various topics, subheadings, images, bibliography and a platform of discussion that reflects responses of visitors to my e-mail address.
1.If cells are intelligent, molecules and their genes would be the 'collaborators' or even 'slaves' , but not the 'masters' of the life functions of cells.
We have all accepted this in the case of organisms. For example, consider the function of an organism like me to make sounds with his throat. Everybody takes it for granted that there is no gene that programmed the words that I speak, but that the information processing speech center in my brain makes the molecules in my throat act and interact when I speak them. Yet, when it comes to cells we tend to believe the opposite: Daily, biologists claim to have found new genes and molecules that act and interact to produce this or the other cell function. If cells are intelligent we would have to rethink all the cause-and-effect chains from genes to molecules to cell function that we believe today to be true.
2. If cells are intelligent, medical treatment may involve 'talking to cells [See ref 17] rather than to flood the organism with pharmaceuticals as we do today.
If cells are intelligent, they are capable of integrating physically different signals (mechanical, electrical, chemical, temperature, pH, etc.) before they generate a response. Integration of physically different signals is only possible if each is first transduced into a common, unifying type of signal. The unifying signals are then integrated and subsequently re-transduced into the response action. For example, all the different kinds of signals that we integrate in our brain are first transduced into unifying electrical pulses, called action potentials, before we integrate them. Finally, we return the same kind of signal, namely electrical response pulses from our brain to the e.g. muscles which re-transduce them into mechanical actions.
If cells have an integrative system, it must also use unifying signals which it links and gates by genetically inherited, cell-specific logical rules before it responds. (Of course, the unknown cellular unifying signals will not be electrical signals like action potentials. Cells are far too small for that. ) In other words, if the cells are intelligent they must use some kind of language which we can learn to imitate.
All diseases are ultimately healed by cells. Doctors 'merely', aid the cells of their patients to do their job. Just imagine, the powerful medicine doctors might practice in the future if they can literally 'tell' cells in their own language what they want them to do! For example, cancer cells might be 'told' to stop growing or at least may be 'summoned' to a certain place on the skin where surgeons can easily scoop them out. Cells at the wound of a lost limb or eye may be 'told' to regrow it. They did it once. If we learn the right 'language' maybe, they can do it again.
3. If cells are intelligent, an organism would be the ecology of a huge population of intelligent individuals.
We tend to believe today that our bodies are highly organized buildings composed of cells which we consider to be dumb miniature machines. Even neurons are treated as complex, yet rather dumb signal switching gadgets. However, if each cell has a certain intelligence to make decisions on its own we would have to reconsider this concept, too. In this case, we would have to look at the structures and functions of our bodies as the result of the interaction of a huge population of intelligent individuals. Possibly, we would have to learn to look at our bodies much the way we consider the complex structures and actions of cities and nations as the result of the actions and interactions of huge numbers of individual people. And 'huge' hardly does justice to the number of cells that make up an organism. For example, one human body has more cells than there would be people on 1000 planet Earths. Also, of course, every cell is a much less intelligent part of a body than a human is part of a city or nation. Still, many of our rather mechanical explanations of body functions would have to be re-examined.